Section.2
Drug discovery research based on individual immunological differences
We aim to establish immunological analysis systems, search for biomarkers, and establish new preventive and therapeutic methods directly related to human clinical applications by comparing human clinical specimens, genetically modified mice as animal models, and non-human primate monkey specimens on a side-by-side basis. Our major research focuses on cancer-related research and infectious disease-related research.
- 1. Developing immunotherapies to achieve an AIDS cure.
- 2. HTLV-1–associated Diseases (ATL/HAM): Immunogenomic and Multi-omics Analysis of Disease States
- 3. Research and Development of Novel COVID-19 Vaccines
- 4. Application to Influenza Vaccine Development
- 5. Development of Dengue Fever Vaccines
- 6. Development of a Therapeutic Vaccine for Hepatitis B Virus Aimed at Achieving a Functional Cure
◆ Infectious Disease Related
1. Developing immunotherapies to achieve an AIDS cure
Human immunodeficiency virus (HIV) is the causative agent of acquired immunodeficiency syndrome (AIDS), and more than 30 years after its identification, approximately 35 million people worldwide remain infected. In Japan, the number of people living with HIV continues to increase and is estimated to have reached approximately 20,000, making HIV/AIDS still one of the most significant emerging and re-emerging infectious diseases.
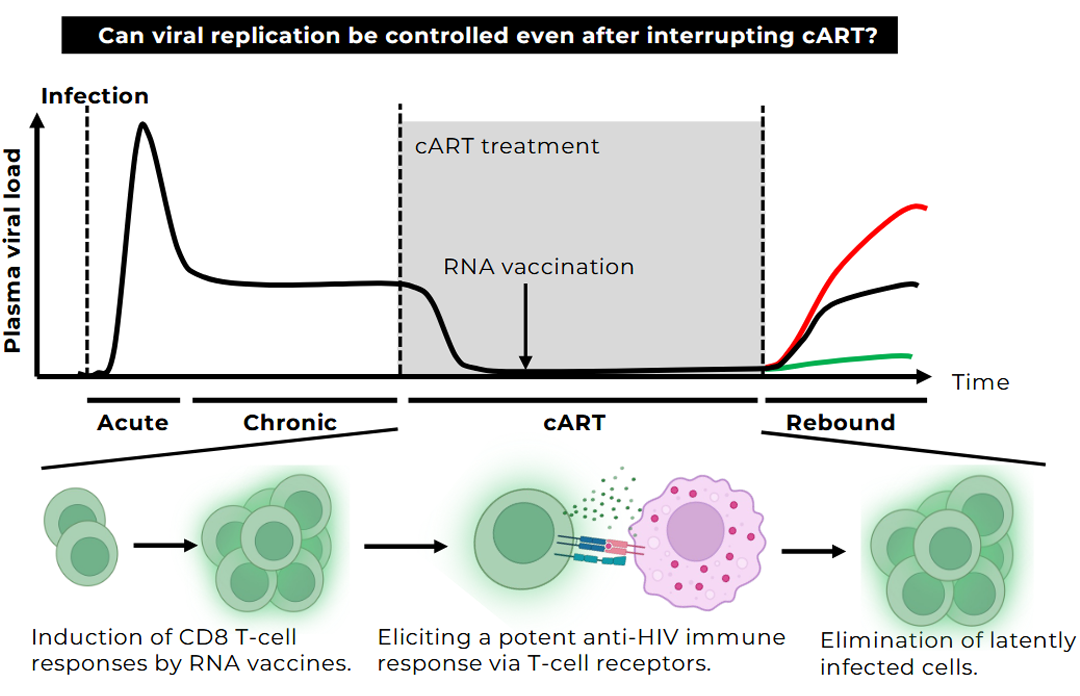
For the treatment of AIDS, multiple antiretroviral drugs have been developed, and the establishment of combination antiretroviral therapy (cART) has made it possible to suppress plasma viral loads to below the limit of detection. However, latently infected cells, referred to as “latent reservoirs,” persist in the body, and viral rebound occurs in most cases when treatment is discontinued. Therefore, achieving a “functional cure,” defined as long-term viral control without continuous drug therapy, requires immunological control and elimination of these latently infected cells.
We are developing a therapeutic RNA vaccine platform centered on the robust activation of CD8⁺ T cell responses. Two key elements underpin this strategy.
1. Antigen design:Based on existing knowledge, we focus on highly immunogenic CD8⁺ T cell epitopes that are resistant to viral escape mutations, and optimize their sequence composition and arrangement.
2. Evaluation of adjuvants combined with mRNA vaccines:To enhance both the magnitude and quality of HIV-specific CD8⁺ T cell responses—such as polyfunctionality and long-term memory formation—we select candidate innate immune agonists as adjuvants and evaluate their combinatorial effects and mechanisms of action using standardized immunological assessment systems.
By integrating these approaches, we aim to efficiently induce HIV-specific CD8⁺ T cells and achieve reduction and functional suppression of the latent reservoir. Taking into account the latest epidemiological data and unmet clinical needs, we seek to develop a novel immunotherapeutic strategy that brings us closer to achieving a functional cure for HIV infection.
Developing immunotherapies to achieve an AIDS cure.
2. HTLV-1–associated Diseases (ATL/HAM): Immunogenomic and Multi-omics Analysis of Disease States
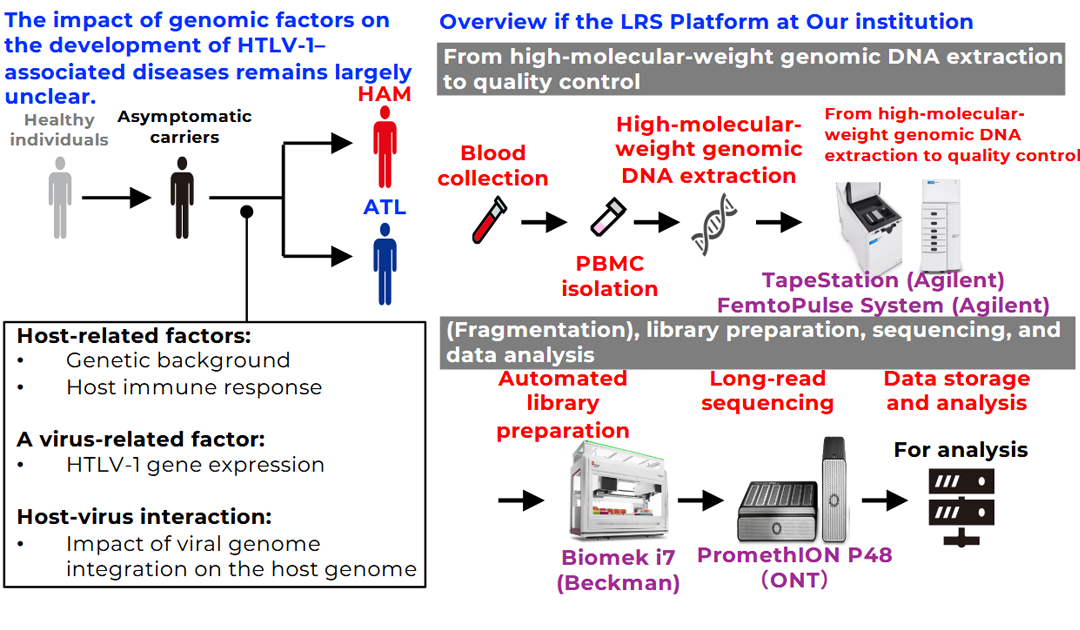
Human T-cell leukemia virus type 1 (HTLV-1) is a virus prevalent in certain regions of the world, including Japan. While a subset of infected individuals develops adult T-cell leukemia/lymphoma (ATL) or a neuroinflammatory spinal cord disease known as HTLV-1–associated myelopathy (HAM), the majority of infected individuals remain asymptomatic throughout their lifetime. The reasons why outcomes differ so markedly among individuals infected with the same virus remain poorly understood.
Recent technological advances have enabled increasingly precise analysis of the human genome. In this study, we will perform whole-genome analyses centered on state-of-the-art long-read sequencing technologies. Conventional sequencing approaches rely on short DNA fragments, which can result in missed or mischaracterized critical genetic alterations. In contrast, long-read sequencing allows DNA to be read in much longer stretches, enabling accurate analysis of complex genomic structures and repetitive regions. This approach provides a unique opportunity to identify previously unrecognized genetic factors involved in the development of HTLV-1–associated diseases.
Furthermore, by integrating long-read sequencing with conventional analytical methods, this study will generate comprehensive datasets and enhance the robustness and reliability of the findings. Through this integrative strategy, we aim to deepen our understanding of disease pathogenesis and to provide insights that may ultimately contribute to risk prediction and the development of novel therapeutic strategies.
Despite the relatively high number of patients with HTLV-1–associated diseases in Japan, the research infrastructure in this field remains insufficient. This study seeks to establish a new analytical platform ahead of both domestic and international efforts, significantly advancing our understanding of HTLV-1 infection and paving the way toward risk stratification and therapeutic development. Ultimately, this research aims to contribute to improved outcomes for patients and to broader societal benefit.
HTLV-1–associated Diseases (ATL/HAM): Immunogenomic and Multi-omics Analysis of Disease States
3. Research and Development of Novel COVID-19 Vaccines
under construction
4. Application to Influenza Vaccine Development
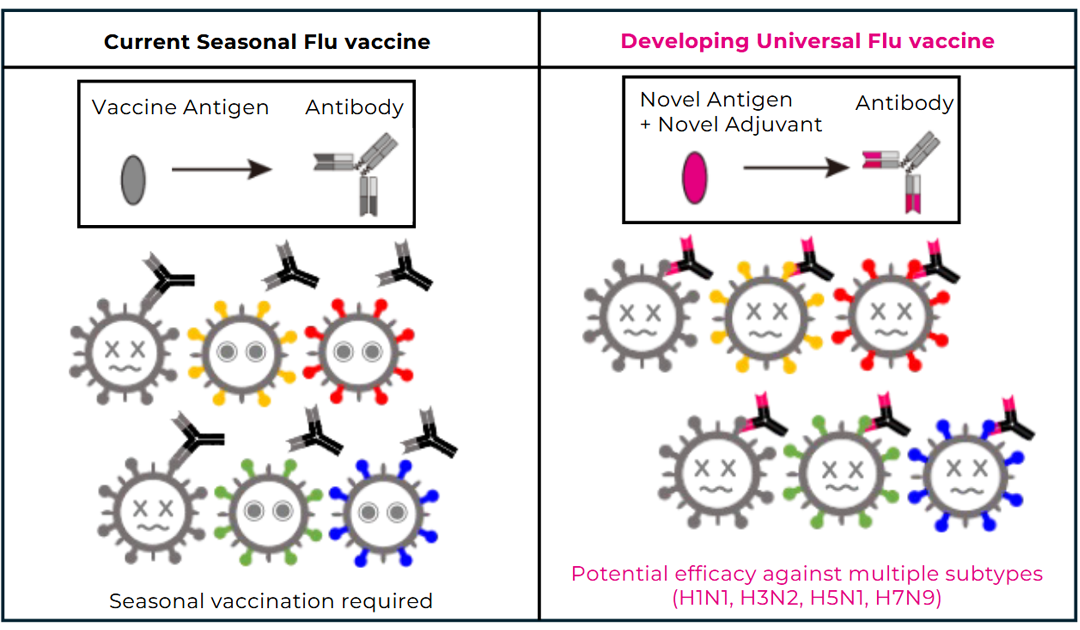
In Japan, more than 25 million doses of seasonal influenza vaccine are produced and administered each year. However, antibodies induced by current vaccines are insufficient to fully cope with the virus’s continuous antigenic drift, necessitating vaccination every season based on circulating strains. Moreover, vaccine efficacy is limited in elderly populations, and in the event of the emergence of a novel virus with antigenic shift—such as during the 2009 influenza pandemic—current vaccines are unlikely to provide adequate protection.
To address these challenges, we analyzed Fc receptor–dependent antibody responses targeting the hemagglutinin (HA) stem region using samples obtained from recipients of seasonal influenza vaccination. We found that vaccination induces HA stem–specific IgG antibodies that correlate with antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). However, the breadth of neutralizing activity was limited, and no cross-neutralization was observed against heterologous subtypes such as H5N1 or H7N9. These findings indicate that while current vaccines can elicit a certain level of immune responses against conserved regions of HA, novel vaccine designs are required to achieve broad cross-protective immunity.
Based on these insights, we are collaborating with academic institutions and pharmaceutical companies in Japan to develop a so-called “universal influenza vaccine” with enhanced cross-protective efficacy. This vaccine candidate has already entered clinical trials in Europe.
Application to Influenza Vaccine Developmen
5. Development of Dengue Fever Vaccines
under construction
6. Development of a Therapeutic Vaccine for Hepatitis B Virus Aimed at Achieving a Functional Cure
Hepatitis B virus (HBV) is an extremely prevalent pathogen, with approximately 250 million people worldwide living with chronic infection, according to World Health Organization estimates in 2022. While a subset of chronically infected individuals progresses to liver cirrhosis or hepatocellular carcinoma, the majority remain asymptomatic, and differences in clinical outcomes are thought to be strongly influenced by host immune responses. Indeed, when immunocompetent adults are newly infected with HBV, most are able to clear the virus through their own immune responses, and the high efficacy of prophylactic vaccination further underscores the critical role of immunity.
Current treatments rely on nucleos(t)ide analogs, which effectively suppress viral replication but do not achieve complete viral eradication. As a result, even with long-term therapy, viral remnants persist within hepatocytes and may contribute to hepatocarcinogenesis. In particular, hepatitis B surface antigen (HBsAg), a key marker of HBV infection, remains detectable in most patients, with spontaneous loss occurring in only a few percent of cases. Persistent HBsAg is associated with an increased risk of future hepatocellular carcinoma, and loss of HBsAg is therefore considered a major clinical treatment goal.
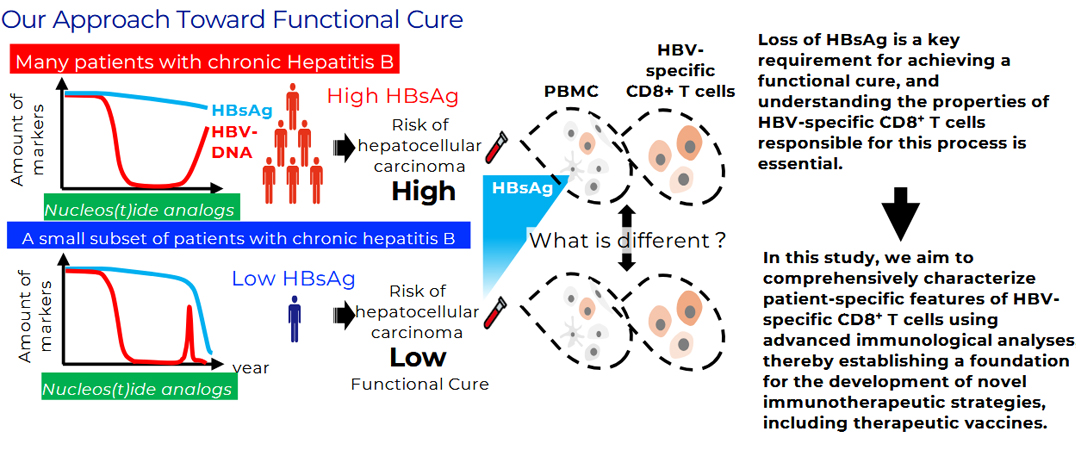
In recent years, because complete viral eradication is difficult to achieve, “functional cure”—defined as sustained viral suppression and resolution of hepatitis mediated by host immunity—has emerged as a new therapeutic goal. Achieving this outcome requires immunological control and elimination of infected cells residing in the liver. We have previously identified that the frequency of a specific subset of HBV-specific CD8⁺ T cells correlates with serum HBsAg levels, suggesting that these cells may serve as an immunological indicator of functional cure. Building on these findings, we are currently developing a novel therapeutic vaccine designed to effectively activate HBV-specific T cells. This vaccine incorporates multiple HBV antigens and is engineered to induce stronger and more durable immune responses.
Development of a Therapeutic Vaccine for Hepatitis B Virus Aimed at Achieving a Functional Cure
◆ Cancer-related
1. Establishment of a Platform for Immunotherapy Development Using mRNA/saRNA Modalities
We are developing a versatile platform based on mRNA/saRNA modalities that enables in vivo expression of antigens or therapeutic proteins and allows rapid redesign according to the target disease or effector molecule. A key feature of saRNA is its ability to self-amplify intracellularly via a replicase, enabling sustained expression over extended periods with relatively low doses. By integratively optimizing sequence design, delivery technologies, and evaluation systems, we aim to achieve durable and highly reproducible expression even at low doses, while carefully assessing the balance between response quality and safety.
This platform is designed with scalability and adaptability in mind, allowing broad applications such as vaccine development and delivery of immunomodulatory molecules. A major strength of the platform lies in its capacity to systematically accumulate and reuse insights obtained at each stage of development as design principles for subsequent iterations. Furthermore, leveraging this versatility, we are also exploring approaches in which antibody genes are expressed in vivo as representative payloads. In this context, key properties such as half-life and effector functions can be “tuned” as needed, offering flexible control over biological activity. Through these efforts, we seek to establish a novel therapeutic option that complements conventional administration of purified antibodies.
2. Development of Novel Immunotherapies for Hard-to-Treat Cancers
Pancreatic cancer is one of the most lethal gastrointestinal malignancies, with a 5-year survival rate of less than 10%. Even with combinations of surgery and chemotherapy, recurrence rates remain as high as 60–70%, underscoring the urgent need for new therapeutic strategies. In recent years, immunotherapies have demonstrated clinical benefit across multiple cancer types, and accumulating evidence suggests that in pancreatic cancer, sustained postoperative immune responses are associated with reduced recurrence. However, pancreatic cancer cells have evolved multiple mechanisms to evade immune surveillance, and overcoming these barriers remains a major challenge. In our laboratory, we are engaged in the development of novel cancer vaccines targeting solid tumors.
Melanoma is a solid malignancy arising in the skin or mucosal tissues, with approximately 325,000 new cases diagnosed worldwide each year, and its incidence is increasing in Japan. Although molecular targeted therapies and immune checkpoint inhibitors have improved outcomes, they are not effective in all patients, highlighting the need for additional therapeutic options. Notably, mRNA vaccine technology, which gained prominence during the COVID-19 pandemic, is highly adaptable to cancer treatment and holds considerable promise as a novel therapeutic approach for melanoma. Building on this technology, our research is focused on two major pillars: (1) selection and validation of shared neoantigens based on large-scale patient datasets, and (2) enhancement of immune responses by combining mRNA vaccines with immunostimulatory molecules. Through these efforts, we aim to advance the clinical application of more effective mRNA-based vaccines for melanoma.
Development of Novel Immunotherapies for Hard-to-Treat Cancers
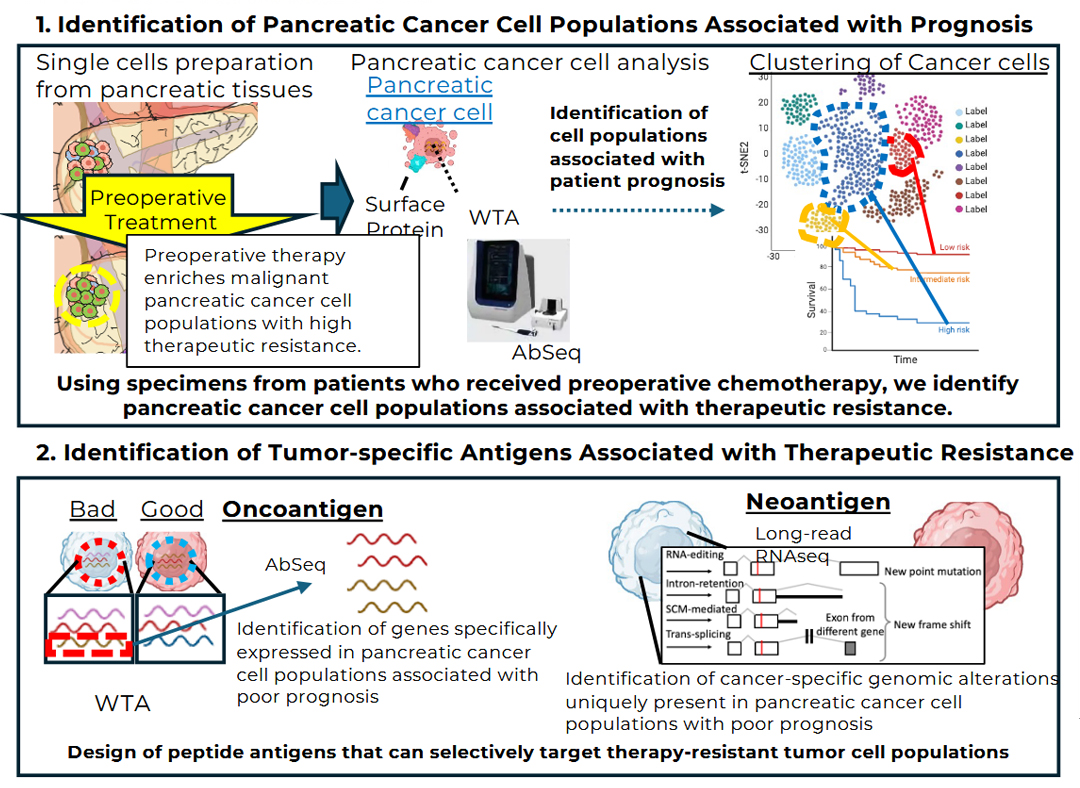
3. Immuno-genomic omics analysis of refractory cancers including pancreatic cancer
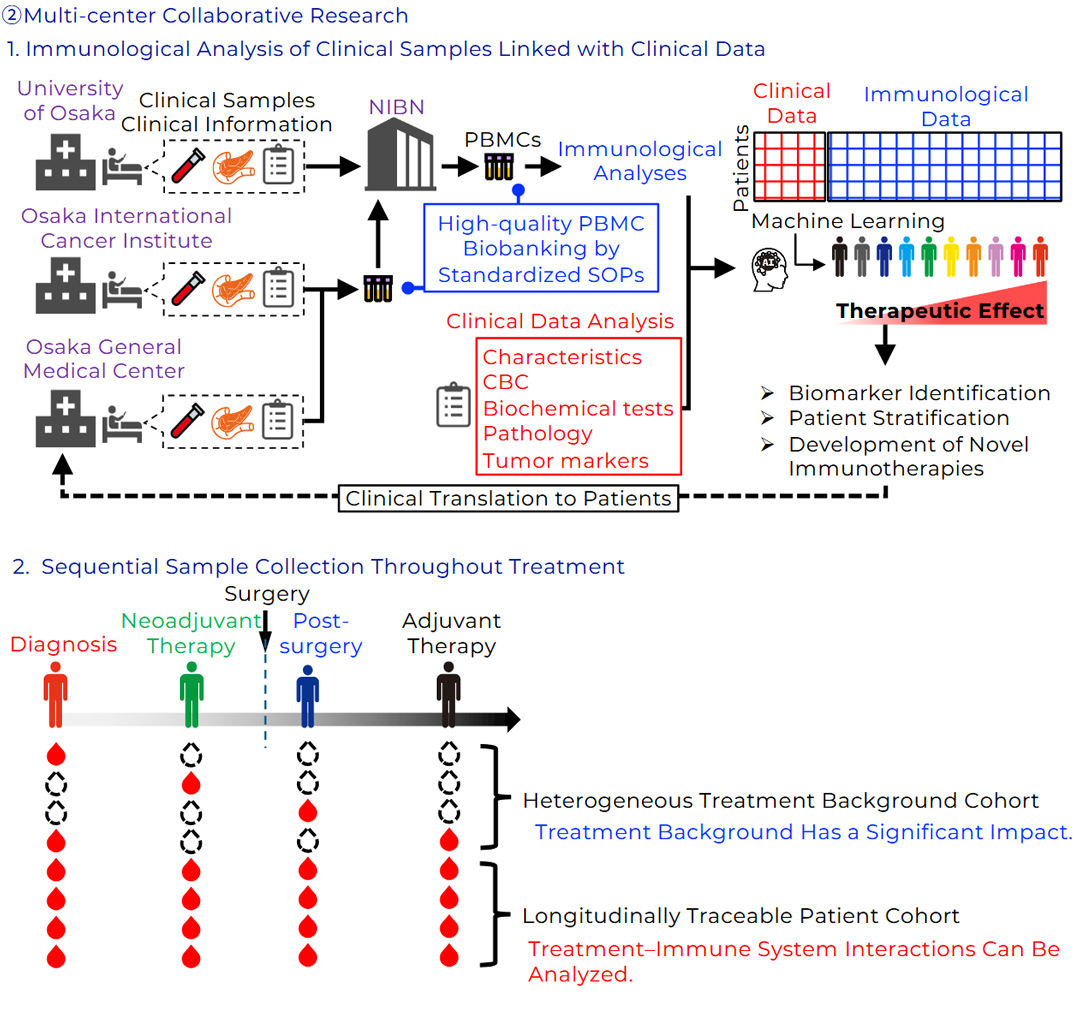
Pancreatic cancer remains an extremely lethal malignancy, with a 5-year survival rate still in the single-digit range. Despite advances in surgical resection and chemotherapy, curative treatment remains difficult to achieve. While immunotherapies, including immune checkpoint inhibitors, are becoming a new pillar of cancer treatment, their efficacy in pancreatic cancer has been limited. Critical unanswered questions remain as to why antitumor immune responses are suppressed in this disease and which tumor cell populations are responsible for therapeutic resistance. Pancreatic cancer is highly heterogeneous, with diverse cell populations coexisting within the tumor tissue. Therefore, single-cell–level analyses of individual tumor cells and the immune cells responding to them are essential for identifying cell populations associated with poor prognosis and treatment resistance.
To address these challenges, we are establishing an integrated multi-omics analysis platform that spans genomic to spatial information. Specifically, pancreatic tumor tissues are dissociated into single cells and analyzed using AbSeq-based surface protein profiling in combination with TCR sequencing (TCR-seq) to characterize the repertoire of tumor-infiltrating T cells, enabling comprehensive assessment of the relationships between tumor cell populations and immune responses. In addition, spatial immune mapping using highly multiplexed immunostaining (CODEX) is performed to visualize the localization and spatial proximity of immune cells and tumor cells, thereby elucidating the structural basis of the tumor microenvironment. Furthermore, long-read RNA sequencing is employed to identify gene expression programs, aberrant splicing events, and candidate neoantigens specific to tumor cell populations associated with poor prognosis, with the goal of discovering novel antigens that may serve as therapeutic targets.
By integrating these complementary analyses, this study aims to precisely identify cell populations that drive therapeutic resistance and poor prognosis in pancreatic cancer and to propose novel immunotherapeutic strategies targeting these populations. Beyond pancreatic cancer, this research framework is broadly applicable to other refractory malignancies and is expected to establish a foundation for personalized immunotherapy for difficult-to-treat cancers.
Immunogenomic and Multi-omics Analysis of Refractory Cancers,Including Pancreatic Cance